Fracture Healing: Understanding the Key Stages and Treatments

Click here to see more
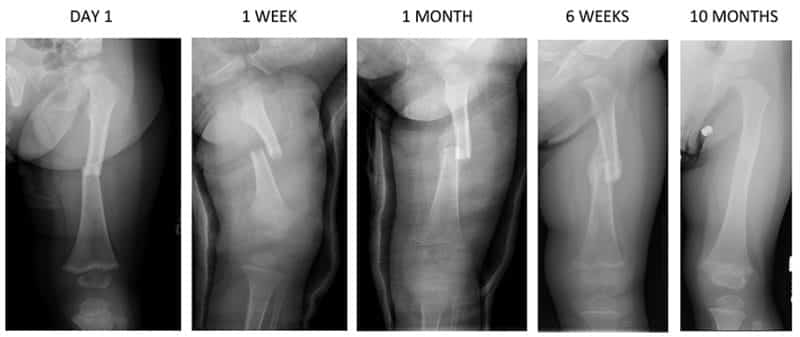
Fracture Healing
Fracture healing involves a complex and sequential set of events to restore injured bone to pre-fracture condition. Stem cells are crucial to the fracture repair process. Periosteum and endosteum are the two major sources. Fracture stability dictates the type of healing that will occur. Mechanical stability governs the mechanical strain. When the strain is below 2%, primary bone healing will occur. When the strain is between 2% and 10%, secondary bone healing will occur.
MODES OF BONE HEALING
Primary Bone Healing (Strain is < 2%)
- Intramembranous healing
- Occurs via Haversian remodeling
- Occurs with absolute stability constructs
Secondary Bone Healing (Strain is between 2%-10%)
- Involves responses in the periosteum and external soft tissues
- Endochondral healing occurs with non-rigid fixation, such as fracture braces, external fixation, bridge plating, intramedullary nailing, etc.
- Bone healing may occur as a combination of the above two process depending on the stability throughout the construct.
TYPE OF FRACTURE HEALING WITH TREATMENT TECHNIQUE
| Treatment Technique | Type of Fracture Healing |
|---|---|
| Cast treatment | Secondary: endochondral ossification |
| External fixation | Secondary: endochondral ossification |
| IM nailing | Secondary: endochondral ossification |
| Compression plate | Primary: Haversian remodeling |
STAGES OF FRACTURE HEALING
Inflammation
- Hematoma forms and provides a source of hematopoietic cells capable of secreting growth factors.
- Macrophages, neutrophils, and platelets release several cytokines:
- PDGF, TNF-Alpha, TGF-Beta, IL-1,6, 10,12
- They may be detected as early as 24 hours post-injury
- Lack of TNF-Alpha (i.e. HIV) results in a delay of both endochondral/intramembranous ossification
- BMPs, fibroblasts, and mesenchymal cells migrate to fracture site and granulation tissue forms around fracture ends.
- During fracture healing, granulation tissue tolerates the greatest strain before failure.
- Osteoblasts and fibroblasts proliferate
- Inhibition of COX-2 (i.e. NSAIDs) causes repression of runx-2/osterix, which are critical for differentiation of osteoblastic cells.
Repair
- Primary callus forms within two weeks. If the bone ends are not touching, then bridging soft callus forms.
- The mechanical environment drives differentiation of either osteoblastic (stable environment) or chondryocytic (unstable environment) lineages of cells.
- Endochondral ossification converts soft callus to hard callus (woven bone).
- Medullary callus also supplements the bridging soft callus.
- Cytokines drive chondrocytic differentiation.
- Cartilage production provides provisional stabilization.
- Type II collagen (cartilage) is produced early in fracture healing and then followed by type I collagen (bone) expression.
- Amount of callus is inversely proportional to extent of immobilization.
- Primary cortical healing occurs with rigid immobilization (i.e. compression plating).
- Endochondral healing with periosteal bridging occurs with closed treatment.
Remodeling
- Begins in middle of repair phase and continues long after clinical union.
- Chondrocytes undergo terminal differentiation.
- Complex interplay of signaling pathways including indian hedgehog (Ihh), parathyroid hormone-related peptide (PTHrP), FGF, and BMP
- These molecules are also involved in terminal differentiation of the appendicular skeleton.
- Type X collagen types is expressed by hypertrophic chondrocytes as the extraarticular matrix undergoes calcification.
- Proteases degrade the extracellular matrix.
- Cartilaginous calcification takes place at the junction between the maturing chondrocytes and newly forming bone.
- Multiple factors are expressed as bone is formed including TGF-Betas, IGFs, osteocalcin, collagen I, V and XI.
- Subsequently, chondrocytes become apoptotic and VEGF production leads to new vessel invasion.
- Newly formed bone (woven bone) is remodeling via organized osteoblastic/osteoclastic activity.
- Shaped through:
- Wolff's law: bone remodels in response to mechanical stress
- Piezoelectric charges: bone remodels in response to electric charges: compression side is electronegative and stimulates osteoblast formation, tension side is electropositive and stimulates osteoclasts
VARIABLES THAT INFLUENCE FRACTURE HEALING
Internal Variables
- Blood supply (most important):
- Initially, the blood flow decreases with vascular disruption.
- After a few hours to days, the blood flow increases.
- This peaks at 2 weeks and normalizes at 3-5 months.
- Un-reamed nails maintain the endosteal blood supply.
- Reaming compromises of the inner 50-80% of the cortex.
- Looser fitting nails allow more quick reperfusion of the endosteal blood supply versus canal filling nails.
- Head injury may increase osteogenic response.
- Mechanical factors:
- Bony soft tissue attachments
- Mechanical stability/strain
- Location of injury
- Degree of bone loss
- Pattern (segmental or fractures with butterfly fragments)
- Increased risk of nonunion likely secondary to compromise of the blood supply to the intercalary segment
External Variables
- Low Intensity Pulsed Ultrasound (LIPUS):
- Exact mechanism for enhancement of fracture healing is not clear.
- Alters protein expression.
- Elevation of vascularity.
- Development of mechanical strain gradient.
- Accelerates fracture healing and increases the mechanical strength of callus (including torque and stiffness).
- The beneficial ultrasound signal is 30 mW/cm2 pulsed-wave.
- Healing rates for delayed unions/nonunions have been reported to be close to 80%.
- Bone stimulators:
- Four main delivery modes of electrical stimulation:
- Direct current: Decreases osteoclast activity and increases osteoblast activity by reducing oxygen concentration and increasing local tissue pH.
- Capacitively coupled electrical fields (alternating current, AC): Affects synthesis of cAMP, collagen, and calcification of cartilage.
- Pulsed electromagnetic fields: Cause calcification of fibrocartilage.
- Combined magnetic fields: Lead to elevated concentrations of TGF-Beta and BMP.
- COX-2: Promotes fracture healing by causing mesenchymal stem cells to differentiate into osteoblasts.
Patient Factors
- Diet:
- Nutritional deficiencies (Vitamin D and calcium).
- As high as 84% of patients with nonunion were found to have metabolic issues.
- Greater than 66% of these patients had vitamin D deficiencies.
- Gastric bypass patients:
- Calcium absorption is affected because of duodenal bypass with Roux-en-Y procedure leads to decreased Ca/Vit D levels, hyperparathyroidism (secondary) & increased Ca resorption from bone
- Diabetes mellitus:
- Affects the repair and remodeling of bone.
- Fracture healing takes 1.6 times longer in diabetic patients versus non-diabetic patients.
- Nicotine:
- Decreases rate of fracture healing.
- Inhibits growth of new blood vessels as bone is remodeled.
- Increases risk of nonunion (increases risk of pseudoarthrosis in spine fusion by 500%).
- Decreased strength of fracture callus.
- Smokers can take ~70% longer to heal open tibial shaft fractures versus non-smokers.
- HIV:
- Higher prevalence of fragility fractures with associated delayed healing.
- Contributing factors:
- Anti-retroviral medication.
- Poor intraosseous circulation.
- TNF-Alpha deficiency.
- Poor nutritional intake.
- Medications affecting healing:
- Bisphosphonates are recognized as a cause of osteoporotic fractures with long-term usage.
- Recent studies demonstrated longer healing times for surgically treated wrist fractures in patients on bisphosphonates.
- Long-term usage may be associated with atypical subtrochanteric/femoral shaft fractures.
- Systemic corticosteroids: studies have shown a 6.5% higher rate of intertrochanteric fracture non unions.
- NSAIDs: Prolonged healing time because of COX enzyme inhibition.
- Quinolones: Toxic to chondrocytes and diminishes fracture repair.
- High dose of radiation: Long-term changes within the remodeling systems (cellularity is diminished).
Click here to see more
Stages of fracture healing
Inflammation
- Hematoma forms and provides a source of hematopoietic cells capable of secreting growth factors.
- Macrophages, neutrophils, and platelets release several cytokines including PDGF, TNF-Alpha, TGF-Beta, IL-1, 6, 10, 12. They may be detected as early as 24 hours post-injury. The lack of TNF-Alpha (ie. HIV) results in the delay of both endochondral/ intramembranous ossification.
- Bone morphogenetic proteins (BMPs), fibroblasts, and mesenchymal cells migrate to fracture site and granulation tissue forms around fracture ends. During fracture healing, granulation tissue tolerates the greatest strain before failure.
- Osteoblasts and fibroblasts proliferate. Inhibition of COX-2 (ie NSAIDs) causes repression of runx-2/osterix, which are critical for differentiation of osteoblastic cells.
Repair
- Primary callus forms within two weeks. If the bone ends are not touching, then bridging soft callus forms.
- The mechanical environment drives differentiation of either osteoblastic (stable enviroment) or chondrocytic (unstable environment) lineages of cells.
- Endochondral ossification converts soft callus to hard callus (woven bone).
- Medullary callus also supplements the bridging soft callus. Cytokines drive chondrocytic differentiation. Cartilage production provides provisional stabilization. Type II collagen (cartilage) is produced early in fracture healing and then followed by type I collagen (bone) expression. The amount of callus is inversely proportional to the extent of immobilization. Primary cortical healing occurs with rigid immobilization (ie. compression plating), while endochondral healing with periosteal bridging occurs with closed treatment.
Remodeling
- Begins in the middle of the repair phase and continues long after clinical union. Chondrocytes undergo terminal differentiation and there is a complex interplay of signaling pathways including Indian hedgehog (Ihh), parathyroid hormone-related peptide (PTHrP), FGF and BMP. These molecules are also involved in terminal differentiation of the appendicular skeleton.
- Type X collagen types is expressed by hypertrophic chondrocytes as the extraarticular matrix undergoes calcification. Proteases degrade the extracellular matrix. Cartilaginous calcification takes place at the junction between the maturing chondrocytes and newly forming bone. Multiple factors are expressed as bone is formed, including TGF-Betas, IGFs, osteocalcin, collagen I, V, and XI. Subsequently, chondrocytes become apoptotic and VEGF production leads to new vessel invasion. Newly formed bone (woven bone) is remodeled via organized osteoblastic/osteoclastic activity.
- Bone is shaped through Wolff's law (bone remodels in response to mechanical stress) and piezoelectric charges (bone remodels in response to electric charges: compression side is electronegative and stimulates osteoblast formation, tension side is electropostive and simulates osteoclasts).
How do bone stimulators help with fracture healing?
Bone stimulators help with fracture healing by delivering electrical stimulation, which decreases osteoclast activity and increases osteoblast activity by reducing oxygen concentration and increasing local tissue pH. There are four main delivery modes of electrical stimulation. Direct current has been used since the 1970s, but it has several limitations, including poor patient compliance due to skin irritation and the need for bulky equipment. Capacitively coupled electrical fields are safe and well-tolerated, but they are much weaker in terms of electrical energy. Pulsed electromagnetic fields are non-invasive and have shown some success, but the mechanisms of action are not fully understood. Finally, low-intensity ultrasound is promising, as it can accelerate fracture healing and increase the mechanical strength of callus (including torque and stiffness). The beneficial ultrasound signal is 30 mW/cm2 pulsed-wave.
How does Low Intensity Pulsed Ultrasound help with bone healing?
Low Intensity Pulsed Ultrasound (LIPUS) can accelerate fracture healing and increase the mechanical strength of callus (including torque and stiffness). The beneficial ultrasound signal is 30 mW/cm2 pulsed-wave.
What kind of bone remodeling occurs with secondary bone healing?
Secondary bone healing (strain is between 2%-10%) involves responses in the periosteum and external soft tissues. It includes endochondral healing.
What kind of bone remodeling occurs with primary bone healing?
Primary bone healing (strain is < 2%) involves intramembranous healing.
What cytokines are released by macrophages, neutrophils, and platelets during the inflammatory stage?
- Platelet-derived growth factor (PDGF)
- Tumor necrosis factor alpha (TNF-alpha)
- Transforming growth factor beta (TGF-beta)
- Interleukin 1 (IL-1)
- Interleukin 6 (IL-6)
- Interleukin 10 (IL-10)
- Interleukin 12 (IL-12)
What forms during the inflammatory stage?
During the inflammatory stage, a hematoma forms and provides a source of hematopoietic cells capable of secreting growth factors. Macrophages, neutrophils, and platelets release several cytokines including PDGF, TNF-Alpha, TGF-Beta, IL-1, 6, 10, 12. They may be detected as early as 24 hours post-injury. The lack of TNF-Alpha (ie. HIV) results in the delay of both endochondral/ intramembranous ossification. Bone morphogenetic proteins (BMPs), fibroblasts, and mesenchymal cells migrate to the fracture site and granulation tissue forms around fracture ends. During fracture healing, granulation tissue tolerates the greatest strain before failure. Osteoblasts and fibroblasts proliferate. Inhibition of COX-2 (ie NSAIDs) causes repression of runx-2/osterix, which are critical for differentiation of osteoblastic cells.

