DEFINITION
■ Compartment syndrome remains one of the most devastating orthopedic conditions. The potential clinical sequelae and medicolegal implications of a possible missed compartment syndrome make it one of the most important entities in all of orthopedic surgery.2
■ Compartment syndrome is a condition, with numerous causes, in which the pressure within the osteofascial compartment rises to a level that exceeds intramuscular arteriolar pressure, resulting in decreased blood flow to the capillaries, decreased oxygen diffusion to the tissue, and ultimately cell death. This is a true orthopaedic emergency.
■ The clinical sequelae of a missed compartment syndrome can be life and limb-threatening. Myonecrosis can lead to acute renal failure and multiorgan failure if not appropriately managed.20
■ Richard von Volkmann first documented nerve injury and subsequent contracture from compartment syndrome in 1872 following a supracondylar fracture.
■ In 1906, Dr. Hildebrand was the first to apply the term “Volkmann ischemic contracture” to define the end result of any untreated compartment syndrome.
■ In 1909, Dr. Thomas described the major causes of compartment syndrome (fractures being the predominant cause) after reviewing the 112 cases published up to that date.
■ The first to suggest that fasciotomy may help prevent contracture was Dr. Murphy in 1914.
■ It was not until 1967 that Seddon, Kelly, and Whitesides described the existence of four compartments in the lower leg and the need to decompress more than just the anterior compartment.
■ Any situation that leads to an increased pressure within the compartment may result in a compartment syndrome.
■ The impermeable fascia prevents fluid from leaking out of the compartment and also prevents an increase in volume that could reduce pressure within the compartment.
■ The incidence of compartment syndrome is 7.3 per 100,000 males and 0.7 per 100,000 females.
■ In those cases, the most common cause was fracture followed by soft tissue injury.
■ McQueen et al found that the incidence of compartment syndrome was nearly equal for both highand low-energy injuries and that open wounds did not decompress the compartments and were not protective.13,14
■ This chapter describes acute compartment syndrome, in contrast to exertional compartment syndrome.
■ Exertional compartment syndrome is a transient chronic condition brought on by exercise. Unlike acute compartment syndrome, exertional compartment syndrome is not an emergency and its treatment is beyond the scope of this chapter.
ANATOMY
■ The lower leg has four compartments: the anterior, lateral, superficial posterior, and deep posterior (FIG 1, Table 1).
■ The anterior compartment is bound anteriorly by fascia, lat-
erally by the anterior intermuscular septum, and posteriorly by the interosseous membrane between the fibula and tibia.
■ The four muscles in this compartment are the tibialis anterior, extensor digitorum longus, extensor hallucis longus, and peroneus tertius.
■ The neurovascular bundle includes the deep peroneal nerve and the anterior tibial artery.
■ The deep peroneal nerve provides sensation to the first dorsal web space of the foot and motor function to all the muscles in the anterior compartment.
■ The anterior tibial artery travels in this compartment just anterior to the tibiofibular interosseous membrane and continues in the foot as the dorsalis pedis artery.
■ The lateral compartment is bordered anteriorly by the fascia, posteriorly by the posterior intermuscular septum, and medially by the fibula.
■ There are only two muscles of the lateral compartment:
the peroneus longus and the peroneus brevis.
■ The major nerve supply to the lateral compartment is the superficial peroneal nerve, which supplies the two muscles of the compartment. The nerve supplies sensation to the dorsum of the foot, except the first dorsal web space.
■ Since the deep peroneal nerve courses proximally around the fibular head, both the deep and superficial peroneal nerves travel within this compartment.
■ There are no main vessels in this compartment, and the muscles receive their blood supply from the peroneal and anterior tibial arteries.
■ The deep posterior compartment contains the flexor digitorum longus, tibialis posterior, and flexor hallucis longus muscles.
■ Although it is not considered a separate compartment, the tibialis posterior muscle can have its own fascial covering.
■ The deep posterior compartment contains the main neurovascular bundle of the posterior compartment, which consists of the tibial nerve, posterior tibial artery and vein, and peroneal artery and vein.
■ The superficial posterior compartment contains the gastrocnemius, soleus, and plantaris muscles, which are supplied by branches of the tibial nerve, posterior tibial artery, and peroneal arteries.
■ There is no major artery that travels in this compartment.
PATHOGENESIS
■ Although the exact pathophysiology is not completely understood, the premise of the syndrome is either a decrease in the space available for the tissues within the fixed compartment or an increase in the size of the tissues within the compartment.
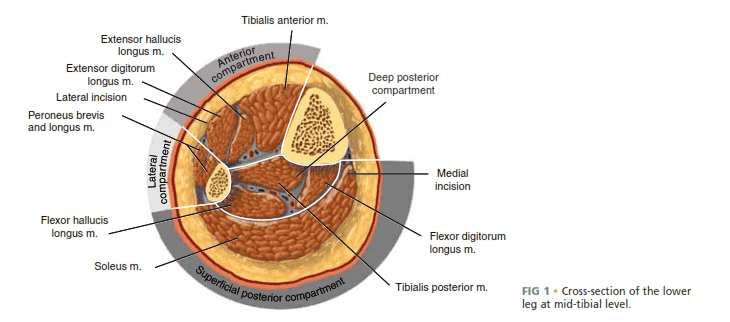
FIG 1 • Cross-section of the lower leg at mid-tibial level.
Table 1 Compartments of the Lower Leg
■ Either case can result in an increase in pressure above a critical value.
■ Increased fluid content and swelling of damaged muscles can be caused by the following:
■ Bleeding into the compartment (from fractures, large vessel injury, or bleeding disorders)
■ Fractures are the most common cause of compartment syndrome. It is estimated that 9.1% of tibial plateau fractures develop compartment syndrome.3
■ Blunt trauma is the second most common cause, accounting for 23% of cases.14
■ Increased capillary permeability (eg, burns, ischemia, exercise, snake bite, drug injection, intravenous fluids)
■ Decreased compartment size can be caused by the following:
■ Burns
■ Tight circumferential wrapping, dressings, casts
■ Localized external pressure, such as lying on the limb for an extended period of time or from pressure on the “well leg” in the lithotomy position on the fracture table
■ Elevated pressure prevents perfusion of the tissue from the capillaries and results in anoxia and necrosis.
■ The impermeable fascia prevents fluid from escaping, causing a rise in compartment pressure such that it exceeds
the pressure within the veins, resulting in their collapse or an increase in the venous pressure.17
■ The final event is cellular anoxia and necrosis.20
■ During necrosis there is an increase in the intracellular calcium concentration coupled with a subsequent shift of water into the tissue, causing the tissue to swell further, adding to the pressure.4 This “capillary leakage” adds to the increased pressure in the compartment, thus creating a vicious cycle.
■ The effects on muscle and nerve function are time-dependent.
■ Prolonged delay results in greater loss of function.
■ After sustained elevation of compartment pressures greater than 6 to 8 hours, nerve conduction is blocked.10 In an animal study, irreversible muscle damage occurred after 8 hours.22
■ The exact pressure at which change within the compartment occurs has been subject to debate and has evolved over time.
■ Initially, the pressure of 30 mm Hg was reported to be the maximum pressure above which irreversible muscle damage occurred.27
■ Currently, clinicians have recognized the importance of the patient’s blood pressure when considering the compartment pressure and use an absolute difference between diastolic blood pressure and compartment pressure of
30 mm Hg as a gauge.
■ Animal studies have highlighted the importance of the systemic pressures relative to the compartment pressure.
■ Heckman et al found that irreversible ischemic changes occurred when the compartment pressure was elevated within 30 mm Hg of the mean arterial pressure and within
20 mm Hg of the diastolic pressure.27
■ Studies on limb ischemia at the University of Pennsylvania had similar conclusions.
■ They coined the term “delta P” referring to the difference between the mean arterial pressure minus the compartment pressure, with a lower number reflecting less blood flow.1
■ They found that cellular anoxia and death occur with pressure within 20 mm Hg of the mean arterial pressure; however, at pressures within 40 mm Hg there was reduced oxygen tension but no evidence of anoxia, and aerobic metabolism persisted.
■ McQueen and Court-Brown used the cutoff of compartment pressure within 30 mm Hg of the diastolic blood pressure as a fasciotomy threshold.13
■ There were no adverse clinical outcomes from not releasing compartments with pressures more than 30 mm Hg from the diastolic blood pressure, and this has come to be the value currently used most often as a threshold for compartment syndrome.
NATURAL HISTORY
■ The outcome of compartment syndrome depends on location and time to intervention.
■ Six hours of ischemia is currently the accepted upper limit of viability. Rorabeck and Macnab reported almost complete recovery of the limb function if fasciotomies are performed within 6 hours of the onset of symptoms.21
■ Muscle undergoes irreversible change after 8 hours of ischemia, whereas nerves can have irreversible damage after as short as 6 hours.10
■ Compartment syndrome may have broad effects on multiple systems.
■ As muscle necrosis occurs, myoglobin, potassium, and other metabolites are released into circulation.
■ As a result, several metabolic conditions can arise, including myoglobinuria, hypothermia, metabolic acidosis, and hyperkalemia. In turn, these biochemical phenomena can cause renal failure, cardiac arrhythmias, and potentially death.
PATIENT HISTORY AND PHYSICAL FINDINGS
■ The use of physical examination findings to diagnose compartment syndrome has not been well validated.26 However, physical examination findings are widely used in clinical practice.
■ The key to successful handling of compartment syndrome is early diagnosis and treatment. Therefore, the orthopedic surgeon must be familiar with the signs and symptoms of the diagnosis and perform a detailed documented history and physical.
■ The patient’s history is critical. Certain aspects of the patient’s history may make the syndrome more likely.
■ The existence of any of the following characteristics should heighten the surgeon’s suspicion: high-energy mechanism, a patient on anticoagulation, or a patient with a tight circumferential dressing.
■ A patient will often not demonstrate all of the classic “six Ps”:
pain, paresthesias, pulselessness, pallor, paralysis, and pressure.
■ Pulselessness has recently been regarded as less of an indicator; patients can suffer extensive compartment syndrome with normal pulses.
■ Likewise, pallor reflects loss of arterial flow and is rarely present on physical examination.
■ Perhaps the most sensitive and earliest sign of compartment syndrome is pain with passive stretch of the muscles of the compartment.4
■ Pain out of proportion to the injury is also an early symptom of the diagnosis.
■ Pain may be absent if compartment syndrome is already established and nerve injury has occurred.
■ Since small fiber nerves are affected first, light touch will be affected before pressure and proprioception.
■ The ability to use pain as an indicator may be diminished in patients unable to sense pain or communicate with the caregivers; in this situation the surgeon must use other means to make the diagnosis.
■ Patients in whom pain may be difficult to ascertain include those with head injuries, those using ethanol or drugs, those who are intubated or sedated, those who have major distracting injuries such as a long bone fracture, those receiving large amounts of pain medicine, or any other factor that might alter the patient’s ability to accurately sense and communicate pain levels.
■ Pain perception may also be altered due to anesthesia, and some reports suggest that patients receiving epidural anesthesia are four times more likely to develop compartment syndrome than those receiving other forms of pain control.19
■ This type of anesthesia results in a sympathetic nerve blockade, thereby increasing the blood flow, compounding the local tissue pressures and extremity swelling.
■ Similarly, local anesthesia combined with narcotics has been shown to increase the risk of compartment syndrome.6,19,20
■ Paresthesia can be a useful, but confusing, symptom of compartment syndrome.
■ It has been shown, however, that nerve function is altered after only 2 hours of ischemia; therefore, it represents a potentially early symptom.8
■ With increased pressure in a compartment, the sensory nerves will be affected first, followed by the motor nerves (eg, in the anterior compartment, the deep peroneal nerve is affected quickly, and patients will report loss of sensation between the first two toes).
■ Paralysis is often less useful since it may be caused by ischemia, guarding, pain, or a combination of these factors, particularly in patients with a distracting extremity injury such as a tibial shaft fracture.
■ Serial examinations are critical. All complaints should be investigated thoroughly, and all findings should be carefully documented in the chart such that subsequent examiners can refer to the record as a tool for diagnosis (see Exam Table for Pelvis and Lower Extremity Trauma, page 1).
■ Measurement of the compartment pressure
■ Stryker pressure monitor is most common (FIG 2).
■ Arterial line (16to 18-gauge needle) is easy to do in the operating room, but the pressure measured with a simple needle is thought to be 5 to 19 mm Hg higher than the pressure measured with a side port or wick catheter.16
■ Pressure values should be recorded for all four compartments. Typically each compartment is checked twice. If there
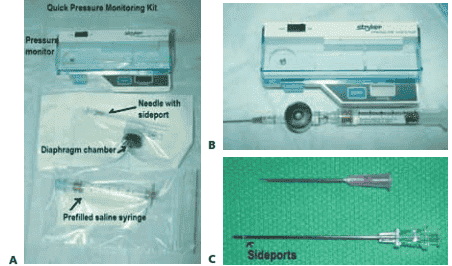
FIG 2 • Stryker intracompartmental pressure monitor. A. Quick pressure monitoring kit containing the intracompartmental pressure monitor, a prefilled saline syringe, a diaphragm chamber (transducer), and a needle. B. The assembled pressure monitor. To assemble the monitor kit, the needle is attached to the tapered end of the tapered chamber stem (transducer). The blue cap from the prefilled syringe is removed and the syringe is screwed into the remaining end of the transducer, which is a Luer-lock connection. The cover of the monitor is opened. The transducer is placed inside the well (black surface down). The snap cover is closed. Next, the clear end cap is pulled off the syringe end, and the monitor is ready to use. To prime the monitor, the needle is held at 45 degrees up from the horizontal and the syringe plunger is pushed slowly to purge air from the syringe. The monitor is then turned on. The assembled monitor is tilted at the approximate intended angle of insertion of the needle into the skin. The zero button is pressed to zero the display. The needle is then inserted into the appropriate location in the compartment. C. The intracompartmental pressure monitor needle has side ports to prevent soft tissue from collapsing around the needle opening. This is different from a regular needle that has only one opening at the end.
is a fracture, the value is checked near and far from the fracture. The contralateral limb can be checked as a control.
■ Delta P (diastolic blood pressure minus intracompartmental pressure) is measured.
■ A delta P less than 30 mm Hg is generally accepted as an indication for fasciotomy. Compartment syndrome is typically a clinical diagnosis and does not need measurement of the pressure before performance of fasciotomy.
■ Compartment pressure checks should be reserved for patients who are difficult to examine, such as sedated patients or those with equivocal examination findings.
■ Tight compartments are an important indicator of compartment syndrome. The deep posterior compartment cannot be palpated directly because of its location deep to the superficial posterior compartment.
■ Pain with passive range of motion is one of the earliest and most important signs of compartment syndrome.
■ Decreased light touch is reported to be one of the first indicators of compartment syndrome. Light touch is a better indicator since it indicates change in the ability of the nerves to detect a threshold force, as opposed to two-point discrimination, which is a test of nerve density and may not change until later in the process.
■ Muscle force should be documented in all compartments when ruling out compartment syndrome. Documenting that patient “wiggles toes” is not adequate as this indicates only
that either the flexor or extensor hallucis longus is firing. “NVI” is also not useful as it does not state the exact muscle groups that were tested.
IMAGING AND OTHER DIAGNOSTIC STUDIES
■ The diagnosis of compartment syndrome is typically made clinically. There are, however, adjunct investigations that can be used to confirm or rule out the diagnosis.
■ Once a patient is diagnosed with compartment syndrome, fasciotomies should be performed emergently. Any workup that could delay this process should be undertaken with great caution.
■ If the patient cannot provide clinical clues because he or she is sedated or for other reasons, or if the diagnosis is in question, compartment pressures can be measured.
■ Several techniques to measure compartment pressures have been described, including the Whiteside infusion technique, the Stic technique, the Wick catheter technique, and the slit catheter technique. The two most commonly used techniques are the Whiteside side port needle and the slit catheter device.
■ There are numerous commercially available digital pressure monitors that are frequently used as well.
■ The exact pressure that defines compartment syndrome is still debatable, although a measured pressure should be taken with reference to the diastolic blood pressure.13
■ McQueen et al did a prospective study on 116 tibial shaft fractures that were monitored continuously for 24 hours. They set the criterion for compartment release at a difference between diastolic and compartment pressures of less than 30 mm Hg. Following this criterion, a total of three had fasciotomies and none of the sample had late sequelae of compartment syndrome 9 months later.
■ The indications to measure compartment pressures include the following: one or more signs or symptoms of compartment syndrome and a confounding factor (eg, local anesthesia), unreliable examination with firmness in an injured extremity, prolonged hypotension and a swollen extremity with firmness, and spontaneous increase in pain after having received adequate analgesia.
■ The technique of measuring compartment pressures must be mastered by the surgeon.
■ Inexperience with the technique may lead to inaccurate data and potentially missed compartment syndrome.
■ When measuring the pressure, the surgeon must be familiar with the local anatomy and able to accurately measure all of the compartments.
■ Location of the measurement is important.
■ Heckman et al reported the highest pressures were within
5 cm of the fracture site; pressures decreased as the measurements were taken distally and proximally to the fracture.27
They recommended that pressures be measured close to and both distal and proximal to the fracture in all compartments. The highest measurement is compared to the diastolic blood pressure and interpreted accordingly.
■ Lab studies should include a complete metabolic profile, a complete blood count with differential, creatine phosphokinase, urine myoglobin, serum myoglobin, urinalysis (which may be positive for blood but negative for red blood cells, indicating myoglobin in the urine due to rhabdomyolysis), and a coagulation profile (prothrombin time, partial thromboplastin time, INR).
■ Obtaining a complete laboratory panel should not delay operative treatment in a diagnosed case of compartment syndrome.
DIFFERENTIAL DIAGNOSIS
■ Compartment syndrome is diagnosed in a patient with either:
■ Suspicious clinical findings as discussed above, or
■ Pressure in a compartment within 30 mm Hg of the diastolic blood pressure
■ Other diagnoses to consider:
■ Normal pain response secondary to fracture or other trauma
■ Low pain tolerance secondary to preoperative substance abuse
■ Muscle rupture
■ Deep venous thrombosis and thrombophlebitis
■ Cellulitis
■ Coelenterate and jellyfish envenomations
■ Necrotizing fasciitis
■ Peripheral vascular injury
■ Peripheral nerve injury
■ Rhabdomyolysis
■ Of special note, in the case of envenomations, recent studies have shown that compartment syndrome is multifactorial and that fasciotomy may not prevent myonecrosis, which may be
due to the direct toxic effect of the venom and the inflammatory response.
■ In these cases, antivenom should be administered; this has been shown to decrease limb hypoperfusion.
NONOPERATIVE MANAGEMENT
■ All patients suspected of having acute compartment syndrome should have emergent fasciotomies performed in the operating room or at the bedside.
■ “Nonoperative” treatment of acute compartment syndrome is never appropriate, as this is a lifeand limb-threatening injury whose successful treatment is based on limiting the time until fasciotomy is performed.
■ Nonoperative treatment of compartment syndrome is reserved for patients presenting very late after a missed compartment syndrome who already have irreversible muscle necrosis.
■ One school of thought is that these patients should not be débrided, as it will only increase the chance of infection.
■ This is controversial and applies only to chronic, missed compartment syndrome.
■ There is consensus that all acute compartment syndromes should be treated operatively with fasciotomies.
■ Since ischemic injury is the basis for compartment syndrome, additional oxygen should be administered to the patient diagnosed with compartment syndrome because it will increase slightly the blood partial pressure of oxygen (PO2).
■ The surgeon must ensure that the patient is normotensive,
as hypotension reduces perfusion pressure and leads to further tissue injury.
■ Any circumferential bandages or casts should be removed in patients at risk for development of compartment syndrome.
■ Compartment pressure falls by 30% when a cast is split on one side and by 65% when a cast is spread after splitting; splitting the padding reduces the pressure by an additional
10%, complete removal of the cast by another 15%. There could be a total of 85% to 90% reduction in pressure by just taking off the cast.28
■ Elevating the limb level above the heart decreases limb mean arterial pressure without changing the intracompartmental pressure. The affected extremity should not be elevated.
■ As shown by Styf and Wiger, after an elevation of 35 cm, the mean perfusion pressure decreased by 23 mm Hg but the intracompartmental pressure stayed the same.28
■ Intravenous fluids should be given to decrease the chance of kidney damage from myoglobin.
■ The “crush syndrome” is a sequela of muscle necrosis (ie, high creatine phosphokinase level, above 20,000 IU) and manifests as nonoliguric renal failure, myoglobinuria, oliguria, shock, acidosis, hyperkalemia, and cardiac arrhythmias.
■ Treatment is supportive, with ventilatory support, hydration, correction of acidosis, and dialysis.
■ It is important in this situation to decrease the metabolic load by preventing ongoing tissue necrosis and débriding all dead tissue.
■ The use of narcotics should be closely recorded and monitored in any patient suspected of having compartment syndrome.
■ The use of local, spinal, or epidural anesthesia for postoperative pain control is generally discouraged in patients at high risk for compartment syndrome as it limits the ability of the clinician to do serial examinations.
SURGICAL MANAGEMENT
■ All patients with acute compartment syndrome should be treated with emergent fasciotomies of the affected compartments, as compartment syndrome is limb-threatening as well as potentially life-threatening if allowed to progress to myonecrosis and renal failure.
■ Time to diagnosis and surgical treatment of compartment syndrome is critical, as nerve damage after 6 hours of ischemia may be irreversible.
■ Patients with compartment syndrome should be given the highest priority and treated as an operative emergency.
■ Fasciotomy of the involved compartment is the standard of care for compartment syndrome.
■ In a trauma setting, typically all four compartments of the leg are released, regardless of evidence of involvement of the other compartments.
■ Fasciotomies should ideally be performed in the operating room.
■ If the patient is too ill to be transported to the operating room or there is no operating room available, fasciotomies can be performed at the bedside in as sterile an environment as possible.
■ The only contraindication to fasciotomy in the face of a compartment syndrome is delayed presentation, in which a patient with missed compartment syndrome presents more than
24 to 48 hours after irreversible injury has set in.
■ Operative treatment is hypothesized to increase infection.
■ It is often difficult to know when a compartment syndrome occurred, however, so in situations in which it is unclear, it is probably wise to release the compartments.
■ One school of thought is that if the compartment syndrome has run its course, fasciotomies should not be performed unless the pressure in the compartment is within 30 mm Hg of diastolic pressure.
■ Fasciotomies are also often performed in a prophylactic manner for any patient with an ischemic limb for more than 6 hours to prevent reperfusion injury.
Preoperative Planning
■ Once compartment syndrome is diagnosed, every effort should be directed at getting the patient to the operating room as quickly as possible for fasciotomies.
■ All further workup should be deferred until fasciotomies are complete, except workup that is needed for a potential life-threatening injury.
■ There is little preoperative planning required for this component of the patient’s treatment.
■ Radiographs should be reviewed to rule out fractures or dislocations; however, additional radiographs can be taken in the operating room after fasciotomies have been completed.
■ Only essential preoperative workup should be done before the patient is taken to the operating room, and the case should certainly not be delayed for additional, nonessential radiographic workup.
Positioning
■ The patient is usually positioned supine on the operating room table to facilitate fasciotomies. A small bump may be placed under the affected hip.
■ The leg is prepared in a sterile fashion and a thigh tourniquet is applied but not inflated.
Approach
■ Two separate techniques have been used for decompression of the lower leg compartments.
■ The two-incision technique is the most commonly used method, but a one-incision technique involving a lateral (perifibular) approach also exists.
■ The two-incision technique is more straightforward and requires less experience to ensure a complete compartment release and therefore is typically advocated.
■ Some have argued that the one-incision technique may be useful in defined anterior tibial artery injuries to help prevent loss of anterior skin.
DOUBLE-INCISION TECHNIQUE
Anterolateral Incision
■ The anterolateral incision decompresses the anterior and lateral compartments.
■ The anterolateral incision is made halfway between
the fibula and the crest of the tibia and lies just above the intermuscular septum dividing the anterior and lateral compartments (TECH FIG 1A).
■ Fasciotomies have also been accomplished through small
incisions. However, we prefer using generous incisions to allow for full decompression of the compartments.
■ We recommend incisions that are typically at least
15 to 20 cm both medially and laterally.
■ A small transverse incision is performed to identify the intermuscular septum, after which scissors are used to split the fascia of the anterior and lateral compartments.
■ Care must be taken to avoid injuring the superficial
peroneal nerve by making separate incisions in each compartment and not cutting the intermuscular septum (TECH FIG 1B–F).
Posteromedial Incision
■ The posteromedial approach decompresses the superficial and deep posterior compartments.
■ The incision lies about 2 cm posterior to the posterior
tibial margin (TECH FIG 2A).
■ Care is taken to avoid injury to the saphenous vein and nerve, which are retracted anteriorly.
■ A small transverse incision is made to allow visualization
of the intermuscular septum between the deep and superficial posterior compartments, after which the fascia of each is incised longitudinally in line with the incision (TECH FIG 2B–E).
■ The deep posterior compartment is initially released dis-
tally, and then the scissors are oriented proximally through and under the soleus bridge.
■ It is crucial to release the soleus attachment to the tibia
more than halfway. Also, the fascia over the posterior tibial muscle should be released.
■ One useful tip is to keep the tips of the scissors away
from major neurovascular structures.
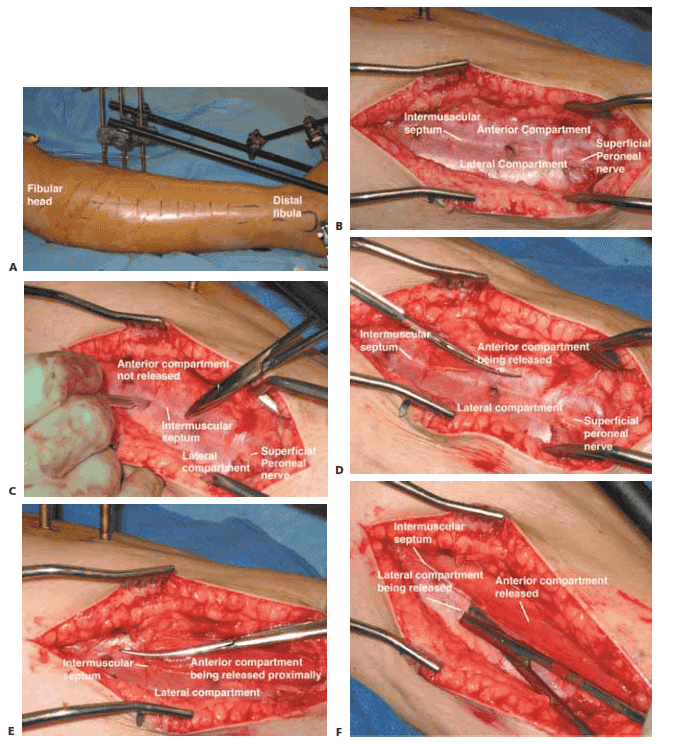
TECH FIG 1 • Lateral incision of the two-incision technique. A. The anterolateral incision is made halfway between the fibula and the tibial crest overlying the intermuscular septum dividing the anterior and lateral compartments. B. Close-up picture of the fasciotomy site after skin incision before fascia is open, showing the intermuscular septum between the lateral and anterior compartments as well as the course of the superficial peroneal nerve. C. With a knife, a small transverse incision is made over the intermuscular septum. Care is taken to avoid injury to the superficial peroneal nerve. D. The surgeon inserts the tips of the scissors into the small rent in the fascia, and keeping the tips of the scissors up and away from the superficial peroneal nerve, the surgeon incises the fascia over the anterior compartment distally. E. The scissors are turned with the tips proximally, and the fascia of the anterior compartment is released proximally. F. The tips of the scissors are then inserted into the rent created in the fascia of the lateral compartment. Keeping the tips of the scissors up and away from the superficial peroneal nerve, the surgeon releases the fascia over the lateral compartment proximal and distal.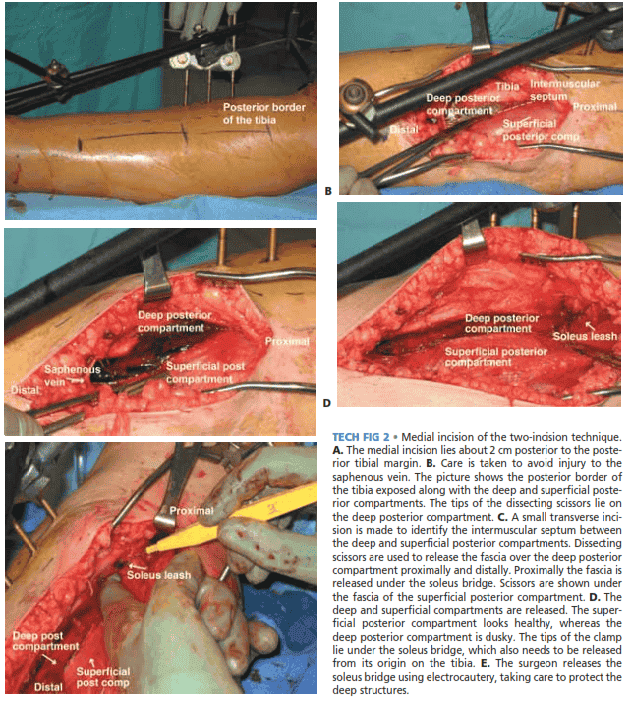
TECH FIG 2 • Medial incision of the two-incision technique.
A. The medial incision lies about 2 cm posterior to the posterior tibial margin. B. Care is taken to avoid injury to the saphenous vein. The picture shows the posterior border of the tibia exposed along with the deep and superficial posterior compartments. The tips of the dissecting scissors lie on the deep posterior compartment. C. A small transverse incision is made to identify the intermuscular septum between the deep and superficial posterior compartments. Dissecting scissors are used to release the fascia over the deep posterior compartment proximally and distally. Proximally the fascia is released under the soleus bridge. Scissors are shown under the fascia of the superficial posterior compartment. D. The deep and superficial compartments are released. The superficial posterior compartment looks healthy, whereas the deep posterior compartment is dusky. The tips of the clamp lie under the soleus bridge, which also needs to be released from its origin on the tibia. E. The surgeon releases the soleus bridge using electrocautery, taking care to protect theىdeep structures
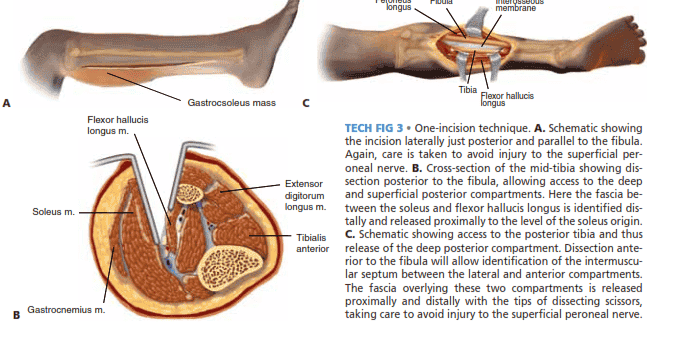
ONE-INCISION TECHNIQUE
■ The one-incision technique often requires more careful dissection around major neurovascular structures and can prove to be more challenging. For this reason it is less often used.
■ A straight lateral incision is created that originates just
posterior and parallel to the fibula at the level of the fibular head (protecting the peroneal nerve) to a point above the tip of the lateral malleolus (TECH FIG 3A).
■ Posterior to the fibula, access is gained to the deep and superficial posterior compartments (TECH FIG 3B).
■ The fascia between the soleus and flexor hallucis
longus is identified distally and released proximally to the level of the soleus origin (TECH FIG 3C).12
■ Anterior to the fibula, the anterior and lateral compart-
ments are decompressed, taking care to avoid injury to the superficial peroneal nerve.
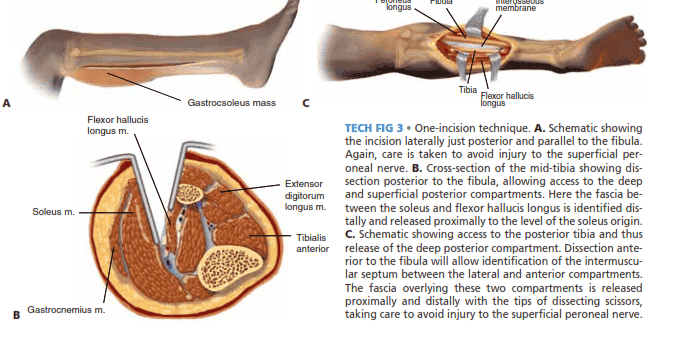
TECH FIG 3 • One-incision technique. A. Schematic showing
the incision laterally just posterior and parallel to the fibula. Again, care is taken to avoid injury to the superficial peroneal nerve. B. Cross-section of the mid-tibia showing dissection posterior to the fibula, allowing access to the deep and superficial posterior compartments. Here the fascia between the soleus and flexor hallucis longus is identified distally and released proximally to the level of the soleus origin. C. Schematic showing access to the posterior tibia and thus release of the deep posterior compartment. Dissection anterior to the fibula will allow identification of the intermuscular septum between the lateral and anterior compartments. The fascia overlying these two compartments is released proximally and distally with the tips of dissecting scissors,
B Gastrocnemius m.
taking care to avoid injury to the superficial peroneal nerve.
MUSCLE DÉBRIDEMENT
■ Regardless of the choice of fasciotomy performed, devitalized muscle is débrided as necessary.
■ Muscle viability is ascertained by the presence of
healthy color and the ability to contract when pinched gently or touched with the electrocautery.
■ Necrotic muscle serves no function and must be re-
moved eventually, as it will form a culture medium for infection after fasciotomy.
■ Extensive débridement is not typically undertaken until
the second look at 36 to 72 hours, when muscle viability is more readily determined.
■ When fasciotomies are performed in the setting of fractures, the fractures are stabilized with either internal or external fixation, which eliminates the need for constrictive casts and allows access for clinical examination, repeat pressure measurements, and wound care.
■ Fixation of fractures may trigger compartment syn-
dromes through traction and reaming.
CLOSURE OF FASCIOTOMIES
■ Fasciotomies are typically not closed acutely because the skin itself can constrict muscle.
■ Most often fasciotomy wounds are either packed with
moist dressings (TECH FIG 4A) or covered with a sterile vacuum sponge and kept under suction until the next débridement (TECH FIG 4B).
■ Following a lower leg fasciotomy, a useful technique has
been the shoelace closure, which involves using a vessel loop and skin staples to gradually close large areas of gaping skin.
■ This allows gradual approximation of the skin edges